We work with partners across the healthcare continuum to co-create innovative medical devices that bring progress to life for patients everywhere.
Services
From spark to solution
Wherever you are in developing your next product, our biomaterial technologies and expertise can help put you on the path to success. Pioneer your next innovation when you tap into the 30+ years of data, IP and expertise of our Biomedical team.
150+ years
Of combined scientific discovery and heritage as part of dsm-firmenich.
30+ years
Of experience working with the world’s leading medical device and pharmaceutical companies.
25 FDA Material Master Files
Available to support regulatory submissions.
Let's build a trusted partnership, together.
We have a deep understanding of how the human body interacts with biomaterials, whether it’s by access or implantation. We’re devoted to working together to create products that are compatible with the body’s physiology, so patients everywhere have access to solutions that sustain, restore, and repair. Whether you’re looking to solve for acute or chronic conditions, we have the biomaterial mastery to chart a clear course to innovation, together.
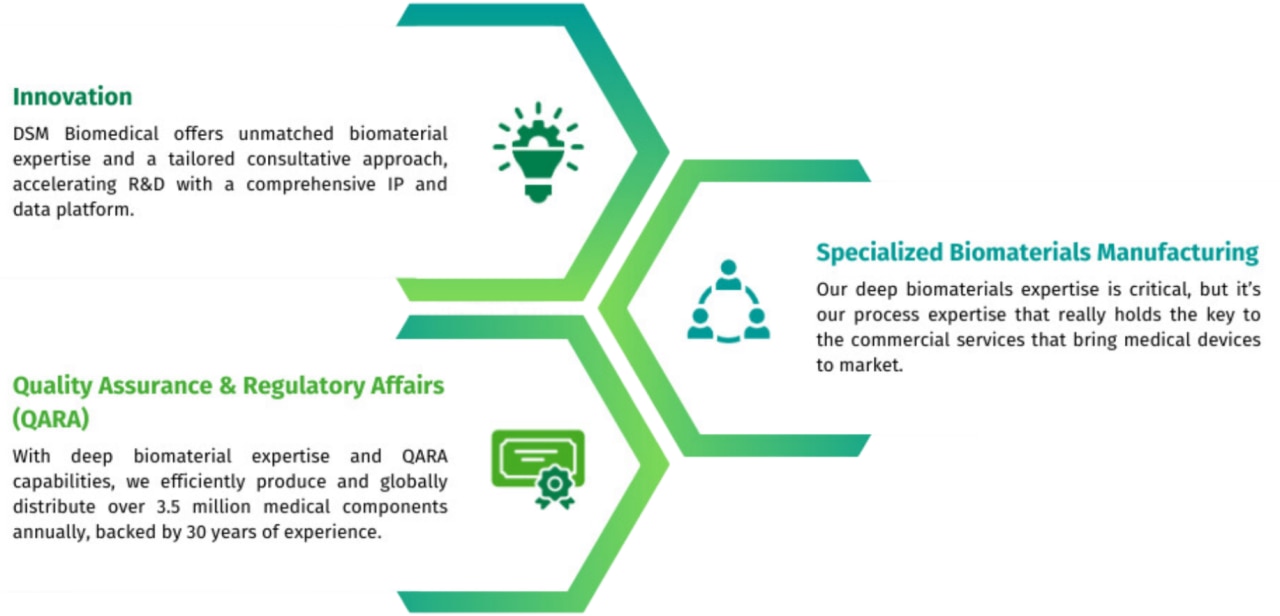
Innovation
Our approach to supporting your innovation is simple. Why reinvent the wheel?
We offer expertise and a wealth of knowledge about biomaterials and how they can be applied and combined to bring your next project to life.
When you innovate with Biomedical:
- De-risk your research & development with our high-quality IP, clinical data and FDA Master files readily on hand.
- You'll have access to our diverse biomaterial portfolio.
- Innovate with a partner dedicated to addressing your development needs from spark to solution.
Quality assurance & regulatory affairs (QARA)
Bring peace of mind to your product development. Our extensive quality system ensures the biomaterials we produce are safe for use in the human body. Our regulatory team works with relevant regulatory bodies to ensure we're meeting stringent requirements and compliance worldwide.
- FDA Master Files on biomaterial technologies can be used as part of your own regulatory submissions. It also includes our first Master File in China for the use of polyethylenes.
- Our ISO 1345 certification can help with the design, development, and manufacturing of biomedical polymers, specialty chemicals, bioresorbable implants fabricated alone or from combinations of synthetic resorbable polymers, bioceramics, and collagen.
- A commitment to best practices. We are regularly audited, and use these findings to further strengthen our quality systems.
Specialized biomaterials manufacturing
Our Specialized Biomaterials Manufacturing (SBM) services are flexible, versatile, and always built on our extensive QARA capabilities, data sets, and IP.
We can support you in scaling up your commercial processes more efficiently through our strategic biomaterial manufacturing capabilities. In fact, our team can transfer entire product lines that we then deliver in a fully packaged and/or sterilized format. Our capabilities here include:
- We manufacture 3.5 million medical components annually from our FDA-certified facility - ranging from less than 50 pieces to over 40,000.
- This is complemented by a 30+ year track record in manufacturing and assembling medical devices (including various U.S. 510K clearances and CE mark approvals).
- From raw materials to fully packaged and sterilized final products, we support you every step of the way with our commercial expertise - all delivered in a turnkey format.
Learn about our biomaterials solutions
-
-
Medical Polyurethanes
-
Natural Biomaterials
-
Polyethylenes
-
Sustained Drug Delivery
-
Biomedical │ dsm-firmenich Health, Nutrition & Care
-
Services
